Good Neural News
Research at the U of A is giving hope to people with Parkinson’s disease.
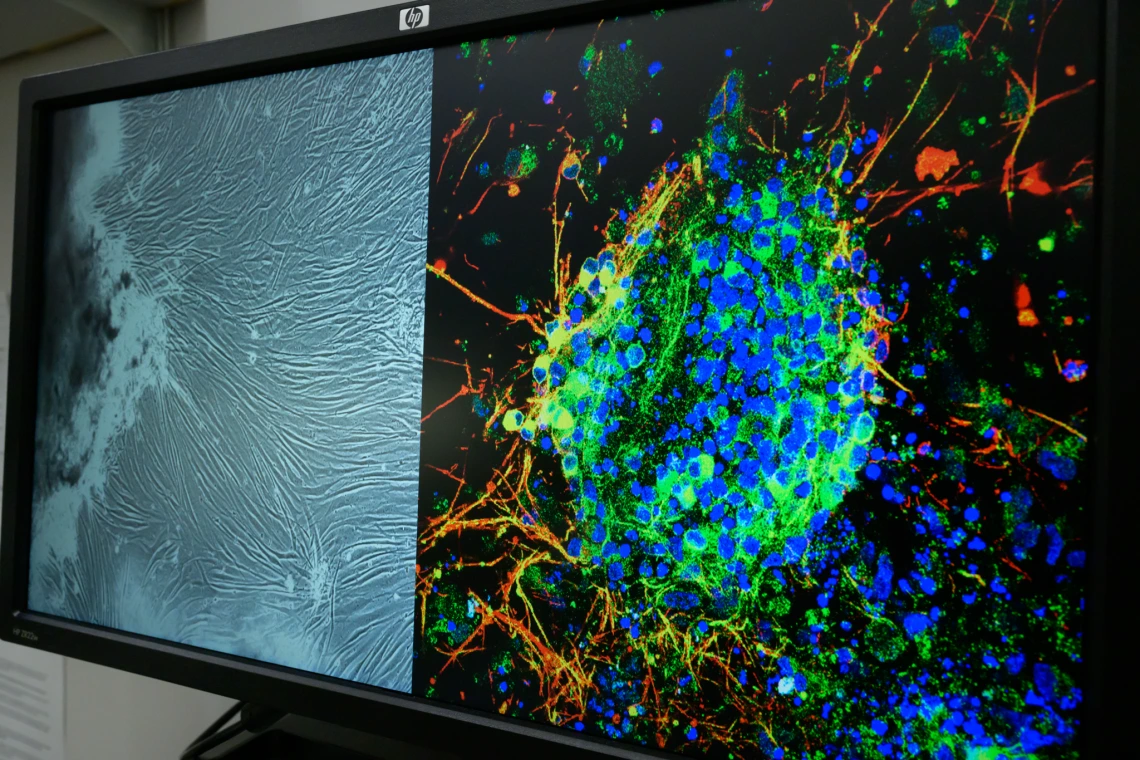
Computer image from the lab of U of A College of Medicine – Tucson researcher Lalitha Madhavan, who uses induced pluripotent stem cell technology to reprogram adult skin cells into brain cells to study Parkinson’s disease.
In the 2010 romantic dramedy “Love & Other Drugs,” starring Anne Hathaway and Jake Gyllenhaal, an old man says of Parkinson’s disease, “It’s not a disease, it’s a Russian novel.” A disease so characterized by slow, long suffering that the screenwriter decided to equate it with the breadth of Tolstoy or the intensity of Dostoevsky.

Stephen Cowen
Bevin Dunn
Parkinson’s disease is the second-most common neurodegenerative disease in the United States. By some estimates, almost 1 million Americans are living with it, including 1% of people over the age of 50.
Parkinson’s disease occurs when neurons in the brain start to die off, causing dopamine, an important brain chemical, to drop dramatically. The decrease in dopamine makes it difficult for patients to move. They experience debilitating tremors, stiffness, pain and cognitive issues such as depression and memory loss.
University of Arizona researchers have published a flurry of research on Parkinson’s in recent months, addressing some of the most understudied and least understood aspects of the disease. Their discoveries are expanding the way doctors think about Parkinson’s disease, even challenging prevailing views on how the disease moves through the brain — all with the goal of pulling patients out of that Russian novel plot.
One of the cruelest ironies of Parkinson’s disease is that the drug best at treating its characteristic rigidity eventually causes its own movement problems. The drug, levodopa, increases dopamine levels in the brain, but when taken chronically, causes involuntary and uncontrollable flailing-like movements, a condition called levodopa-induced dyskinesia. Nearly 40% of patients develop dyskinesia after four to six years on the drug.
One of the U of A studies showed that a single dose of ketamine could reduce these flailing movements in rats and reduce patterns of pathological brain activity associated with Parkinson’s disease and dyskinesia. The study was conducted by Torsten Falk, Ph.D., a professor of neurology at the U of A College of Medicine – Tucson, Stephen Cowen, Ph.D., associate professor in the Department of Psychology at the College of Science, and Abhilasha Vishwanath, Ph.D., a postdoctoral research associate, also at the Department of Psychology.

Abhilasha Vishwanath
College of Science
Rats are a common experimental subject for investigating Parkinson’s disease, as they share the same brain structures that are most affected in human patients. In Vishwanath’s study, rats had their dopamine neurons knocked out to model the cell death in Parkinson’s disease. These animals were then treated with levodopa, which triggered the disordered flailing movements seen in human patients with dyskinesia. These movements were then reduced by ketamine.
What the researchers found challenged the existing view of how these uncontrollable movements are created. It was previously believed that the motor cortex, a part of the brain that controls movement, sent faulty signals to cause the unusual movements. But Vishwanath and Cowen’s team found a disconnect between the activity of neurons in the motor cortex and the movements themselves.
“A healthy motor cortex is conducting and organizing and synchronizing movements,” Cowen says. “But [for these patients] that conducting role seems to disappear. The conductor leaves the building, and these movements are just left to do what they want on their own. And as a result, you get the emergence of these uncontrolled movements.”
“[The motor cortex] is failing to send timely stop signals,” Vishwanath adds.
Ketamine, already known for its neuroplastic effects for people with depression, changes the activity in the brain and resets it.
How ketamine works to reduce uncontrolled movements is not known, but this study provides some clues. Specifically, Cowen and his team noticed that ketamine radically reorganized the interactions between motor cortex neurons and eliminated a high-frequency brain wave associated with the disease. Like shaking a bucket full of marbles, ketamine may act to randomize and reorganize brain activity, derailing pathological patterns and helping the motor cortex regain control of movement.
“[Levodopa] works amazingly well for years,” Cowen says, “so, we’d love to have these patients take the drug longer.”
Levodopa is still the best treatment for Parkinson’s disease, and ketamine might be the key to letting patients reap its benefits without the chronic side effects. This treatment is being explored in human patients by College of Medicine – Tucson neurology professor Scott Sherman, M.D., Ph.D., who helped organized a successful Phase 1 clinical trial and is planning a Phase 2 clinical trial.
This research was supported by grants from the Arizona Biomedical Research Commission to Falk and Sherman; from the National Institutes of Health NINDS to Falk and Cowen; from the Robert and Payton Davies Parkinson’s Disease Research Fund; from the Jerry T. and Glenda G. Jackson Fellowship in Parkinson’s Research; and an estate gift by Mrs. Mary Mack to Sherman.

Kelsey Bernard
Kris Hanning
The movement challenges caused by Parkinson’s disease have long been the most visible and, therefore, the primary focus of treatments. The disease’s cognitive symptoms receive less attention and currently lack effective treatments.
“Motor [symptoms are] the most obvious and the most initially debilitating,” says Kelsey Bernard, Ph.D., a postdoctoral researcher. “But we currently have a drug that alleviates their motor symptoms. So, once you’ve got that under control, then you can see these other issues that are not addressed.”
Bernard conducts her research under the mentorship of Lalitha Madhavan, M.D., Ph.D., who is co-principal investigator along with Falk, both professors of neurology at the College of Medicine – Tucson. Their research programs focus on Parkinson’s disease progression and developing much-needed therapeutics for this disorder.
Bernard’s study focused on a way to protect the brain from the neurodegeneration that occurs with Parkinson’s disease, building on the known neuroprotective effects of a tiny protein called PNA5.
Reduced dopamine seems to be the primary driver behind the stiffness and rigidity associated with Parkinson’s disease. But it’s the chronic inflammation of the brain driven by brain cells called the microglia that could contribute to the cognitive issues Parkinson’s patients experience. Just as a sprained ankle triggers inflammation to protect injured tendons, the microglia in Parkinson’s patients release inflammatory chemicals in the brain. According to Bernard, while this inflammation may offer short-term protection, chronic exposure to these inflammatory chemicals leads to neurodegeneration.
“If [ankle] swelling continued for three weeks out, and there was no physiological need for that to occur, that causes subsequent issues,” she says.
She found that PNA5 calmed the microglia’s overactive immune response, lowering inflammatory chemical levels in the blood of mice in the study. And she observed not just chemical changes but also shifts in the animals’ cognitive behavior. The mice were tested in a maze and in an object recognition task — both standard methods for assessing cognition in lab animals, which correlates with human cognitive function.

Torsten Falk and Scott Sherman
Kris Hanning
“PNA5 completely restored the deficit seen in the Parkinson’s animals,” she says. The mice with Parkinson’s disease were just as good at navigating the maze and picking objects as healthy mice. And she didn’t see any improvement in the motor issues, narrowing PNA5’s effects to cognition only.
A major advantage of PNA5 is that it can be injected anywhere in the body and still cross into the brain, eliminating the need for direct brain injections and making the treatment far more accessible. Bernard is also hopeful that an oral version will prove as effective as the injectable form she tested.
This research was supported in part by a grant from the Michael J. Fox Foundation for Parkinson’s Research to Falk and Madhavan; from the National Institutes of Health; from the ARCS Foundation Scholarship to Bernard; and from the Robert and Payton Davies Parkinson’s Disease Research Fund to Sherman and Falk.
But what if we could get patients off drugs completely? Paul Larson, M.D., professor of neurosurgery at the College of Medicine – Tucson and chief of neurosurgery at the Southern Arizona VA Health Care System, performed a first-of-its-kind surgery in 2024 when he implanted healthy neurons into the brains of Parkinson’s patients. The goal was to replace the dopamine neurons lost to Parkinson’s in a clinical trial sponsored by Aspen Neuroscience.
Larson used cells taken from the patients’ own skin that had been transformed into immature dopamine-releasing neurons. He then surgically deposited them into the patient’s brain.
“We believe they’ll integrate into the circuit, and they’ll start producing dopamine,” he says. “Over time, they’ll actually become part of the circuit.”
One of the key breakthroughs of this treatment is its use of the patient’s own cells, which Larson hopes will eliminate the need for the immune-suppressing drugs typically required for donor transplants. The body attacks foreign cells, forcing transplant recipients to take lifelong medication to prevent rejection. Because Larson’s patients are using their own modified cells, the hope is that these drugs won’t be necessary.

The lab of Lalitha Madhavan (right)
Kris Hanning
While it’s too early to start evaluating results from the Aspen trial, he is hoping the treatment works just as well as or better than one of the traditional surgical interventions for Parkinson’s, deep brain stimulation (DBS). DBS involves implanting electrodes into the brain that act like a pacemaker for the brain — their mild electric pulses keep the electricity in the brain on track and reduce motor symptoms for Parkinson’s patients.
“With DBS, there’s a significant ongoing maintenance requirement: wiring, a battery that needs recharging or replacing,” he says. “You have to go in and have the settings adjusted as your disease progresses.
Larson hopes the transplanted cells are just as effective as DBS, without the need for upkeep. That might help write a whole new story.

Paul Larson
Kris Hanning
Interested in Learning More?
University of Arizona Health Sciences
Study identifies potential new drug for Parkinson’s-related cognitive decline, dementia
